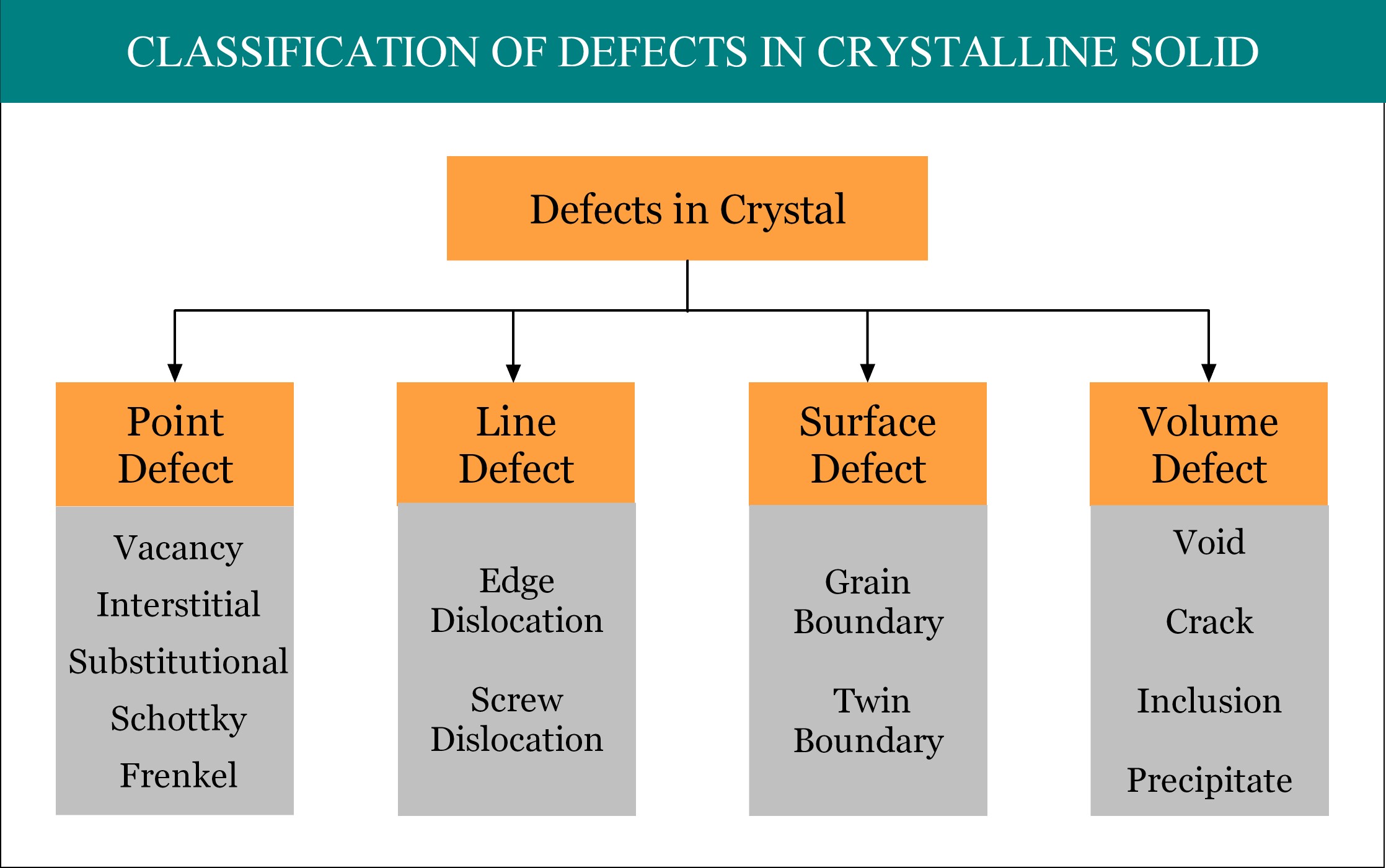
Imperfections or defects in crystalline solid can be broadly classified into four groups, namely, point defect, line defect, surface defect and volume defect. Point defect is considered as the zero dimensional (0-D) defect, as by mathematical definition, a point is unit-less dimensionless quantity! A point defect occurs when one or more atoms of a crystalline solid leave their original lattice site and/or foreign atoms occupy the interstitial position / lattice site of the crystal. There are several types of point defects and vacancy is one of them.
Concept of crystalline solid:
Crystalline solids are those where atoms or molecules or ions are arranged in a regular pattern (known as crystal lattice) that extends in all directions. So crystal lattice is a highly ordered three-dimensional structure of atoms or molecules or ions. If the arrangement of atoms or molecules or ions is highly irregular, then the solid is termed as Amorphous. In between there exist Polycrystalline solids, where few different crystal lattices are observed within same solid. Diamond and Salt are common example of crystalline solid; whereas, Wax, Glass, etc. are amorphous.
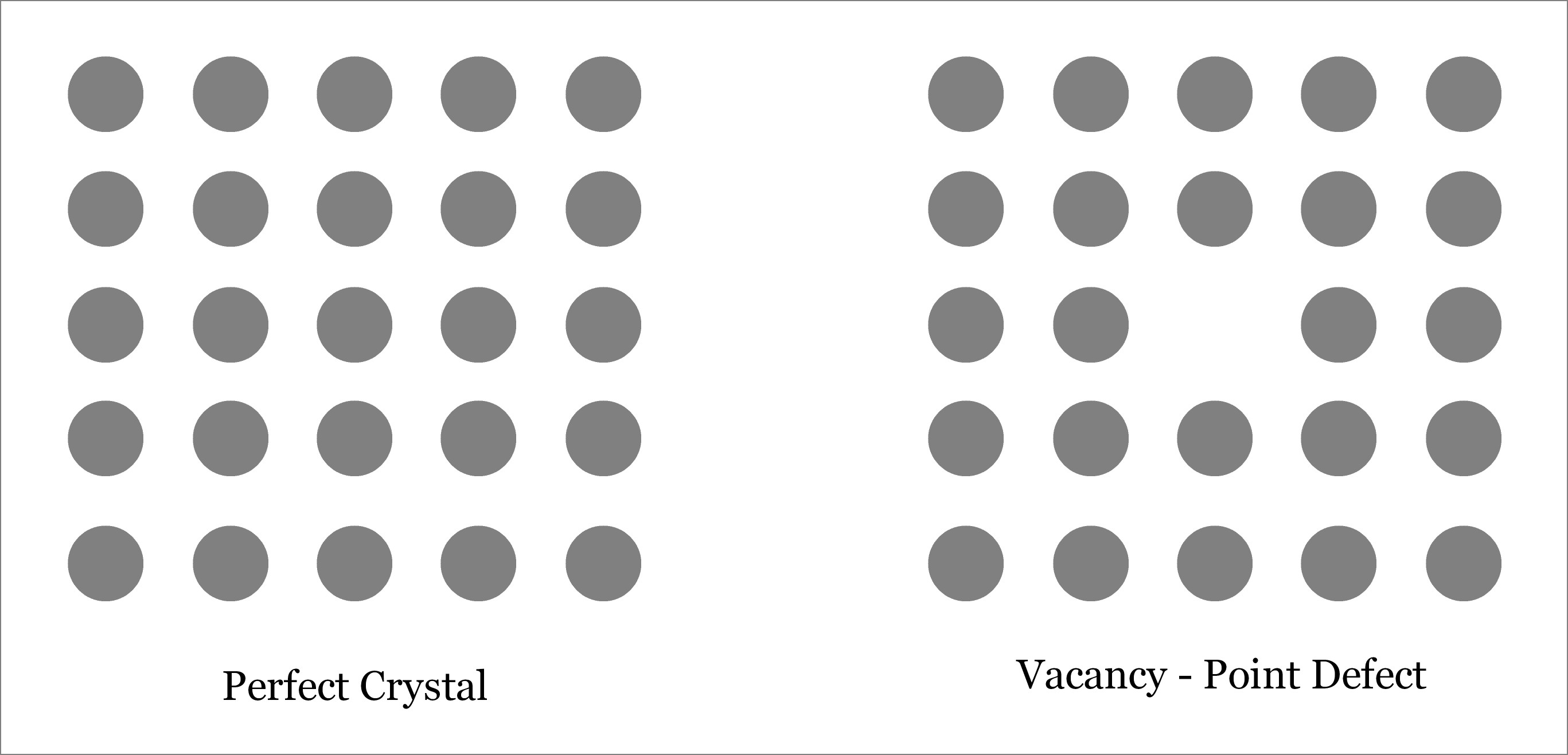
What is vacancy defect in crystalline solids?
A vacancy is produced when an atom is missing from its original lattice site. So vacancy creates an empty lattice site as depicted below. Like other point defects, vacancy is also a zero-dimensional defect. Vacancy defect puts the neighboring atoms under tension. Due to the reduction in number of atoms in the crystalline solid, vacancy defect results in the reduction of density. However, hardness of the solid may increase.
Where vacancy defects can be found?
Vacancy defect can occur in any crystalline solid, in fact, it is inherent. Any material whose temperature is above absolute zero temperature (0K), can contain vacancies. With increase in temperature, number of vacancy defects increases exponentially.
Causes of vacancy defects in solids:
Vacancy, a point defect, may occur due to various reasons, as enlisted below:
- For not allowing directional solidification, usually during casting.
- Due to increase in temperature of the solid for various processing like heat treatment, coating, etc. Number of vacancies in a specific amount of solid increases exponentially as indicated by the expression provided below.
- Due to irradiation or sputtering effect.
- Due to presence of residual tensile stress within the solid.
Effects of vacancies in solid:
- Although depends on material and its crystal structure, in general, vacancies can decrease the bulk modulus and can increase the Young’s modulus.
- Large vacancy concentration can reduce the ductility of the crystalline solid; however, can increase the hardness.
- It can alter the thermal and electrical resistivity of the solid.
- Common physical properties, like melting point, color, etc. can also vary due to presence of vacancies.
Calculation of number of vacancies in solid:
Vacancy defects are temperature sensitive. The number of vacancies present within a particular volume of solid at a particular temperature can be mathematically expressed by the following expression:
$${N_v} = N \times {e^{\left( {{{ – {Q_v}} \over {RT}}} \right)}}$$
Nv = Total number of vacancies per unit volume of solid at a particular temperature (vacancies/m3).
N = Total number of lattice sites per unit volume of solid (lattice sites/m3).
Qv = Energy required to produce single vacancy in that solid (J/mole).
R = Gas constant = 8.314 J/mole-K
T = Absolute temperature of the solid (K)