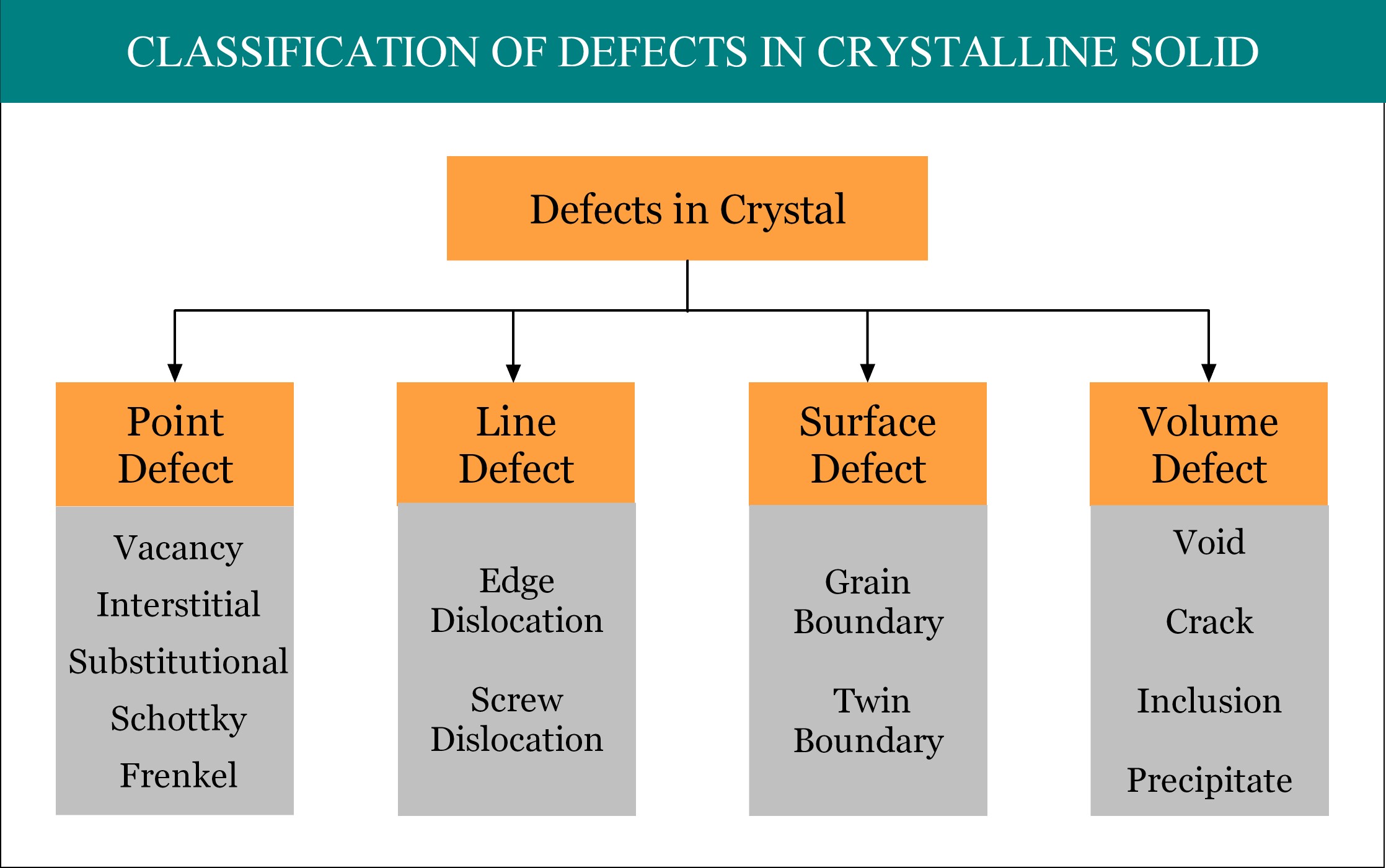
Imperfections or defects in crystalline solid can be broadly classified into four groups, namely, point defect, line defect, surface defect and volume defect. Point defect is considered as the zero dimensional (0-D) defect, as by mathematical definition, a point is unit-less dimensionless quantity! A point defect occurs when one or more atoms of a crystalline solid leave their original lattice site and/or foreign atoms occupy the interstitial position / lattice site of the crystal. There are several types of point defects and Substitutional defect is one of them.
Concept of crystalline solid:
Crystalline solids are those where atoms or molecules or ions are arranged in a regular pattern (known as crystal lattice) that extends in all directions. So crystal lattice is a highly ordered three-dimensional structure of atoms or molecules or ions. If the arrangement of atoms or molecules or ions is highly irregular, then the solid is termed as Amorphous. In between there exist Polycrystalline solids, where few different crystal lattices are observed within same solid. Diamond and Salt are common example of crystalline solid; whereas, Wax, Glass, etc. are amorphous.
What is Substitutional defect in solid?
Substitutional Defect occurs when the original atom in the lattice site of a crystalline solid is replaced by a different type of atom. Unlike interstitial defect, foreign atom should occupy the lattice site only and not the interstitial position, as depicted below. The foreign atom may be of same size or different (either larger or smaller). Depending on the size of the substituted foreign atom, the neighboring atoms may remain either in tension or in compression.
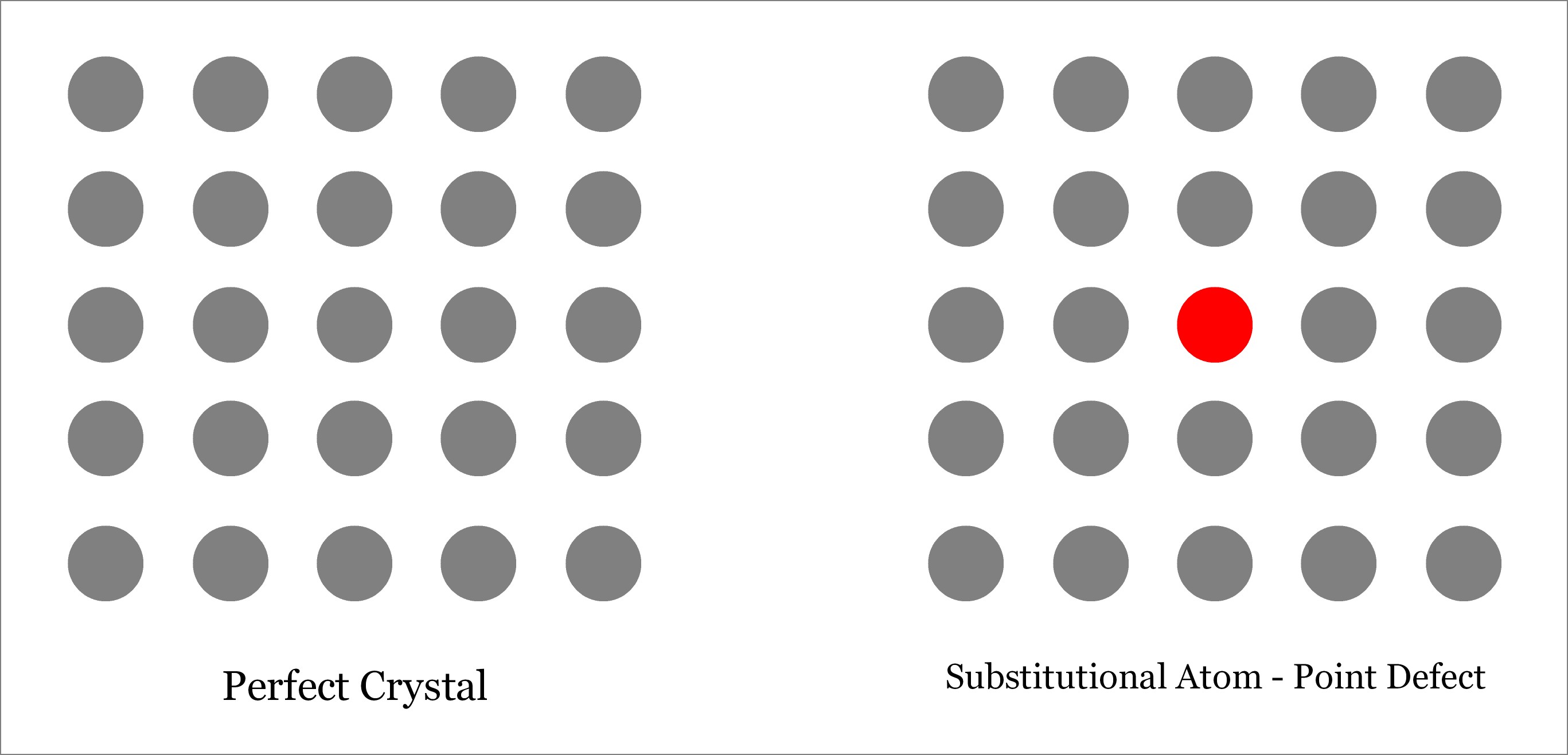
Where Substitutional defects can be found?
- Substitutional defects can be found in brass, where zinc atoms replace copper atoms.
- In semiconductor materials also.
Causes of Substitutional defect in solids:
- Presence of foreign atoms as natural impurities within the solid.
- Deliberate addition, such as during iron to steel conversion, heat treatment, sputtering, etc.
- Diffusion, caused by close contact between two different materials.
Effects of Substitutional defects in solids:
- If the foreign atom is smaller in size as compared to the original atom of the solid, the neighboring atoms will remain in tensile stress.
- If the foreign atom is larger in size as compared to the original atom of the solid, the neighboring atoms will remain in compressive stress.
- So, presence of foreign atom may distort the original lattice structure.
- Presence of substantial number of foreign atoms can change the mechanical and thermal properties of the solid. However, this is sometime beneficial, and thus substitutional defects can be applied in a controlled way to enhance various properties of the solid.