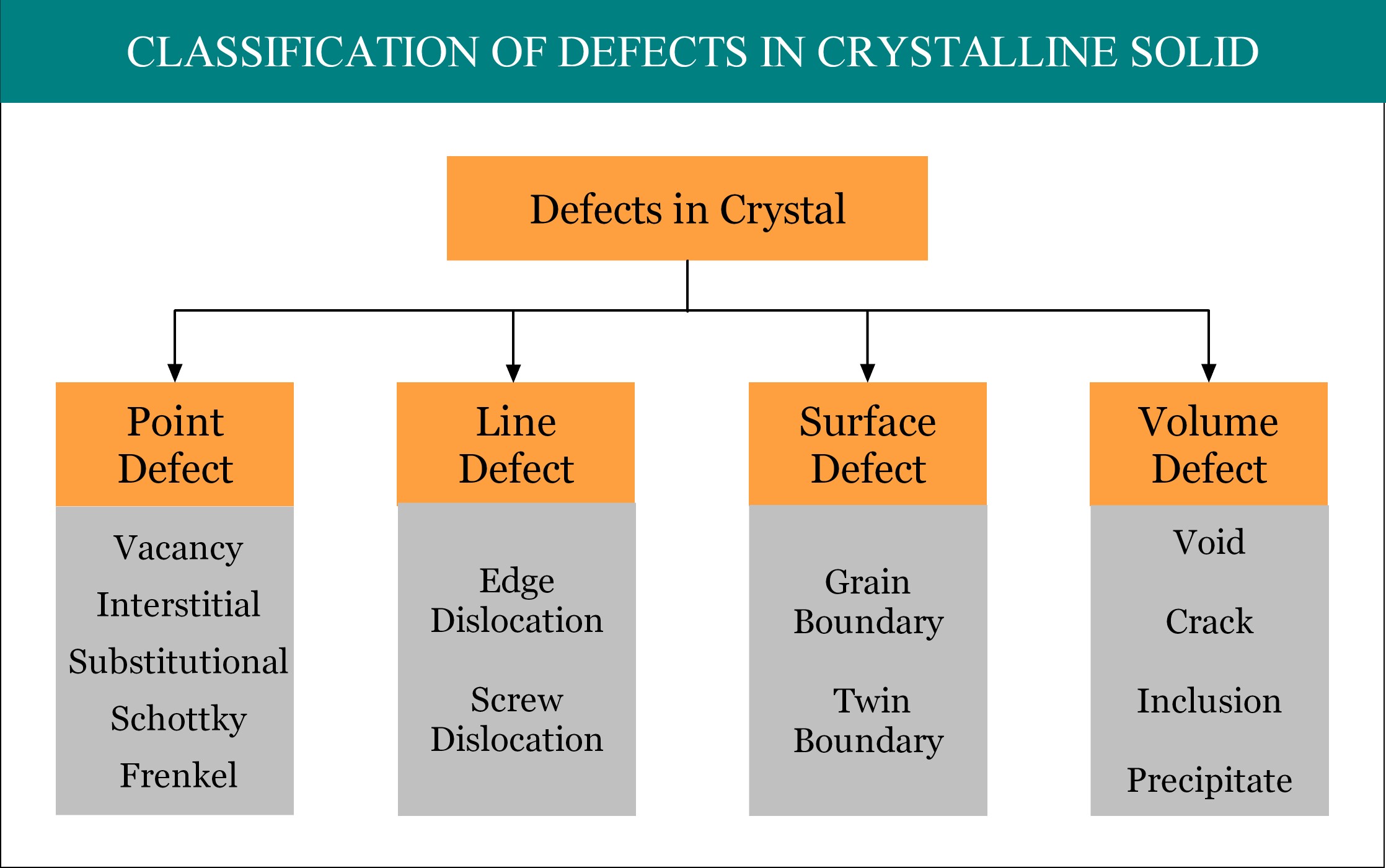
Imperfections or defects in crystalline solid can be broadly classified into four groups, namely, point defect, line defect, surface defect and volume defect. Point defect is considered as the zero dimensional (0-D) defect, as by mathematical definition, a point is unit-less dimensionless quantity! A point defect occurs when one or more atoms of a crystalline solid leave their original lattice site and/or foreign atoms occupy the interstitial position / lattice site of the crystal. There are several types of point defects and Frenkel Defect is one of them.
What is Frenkel Defect?
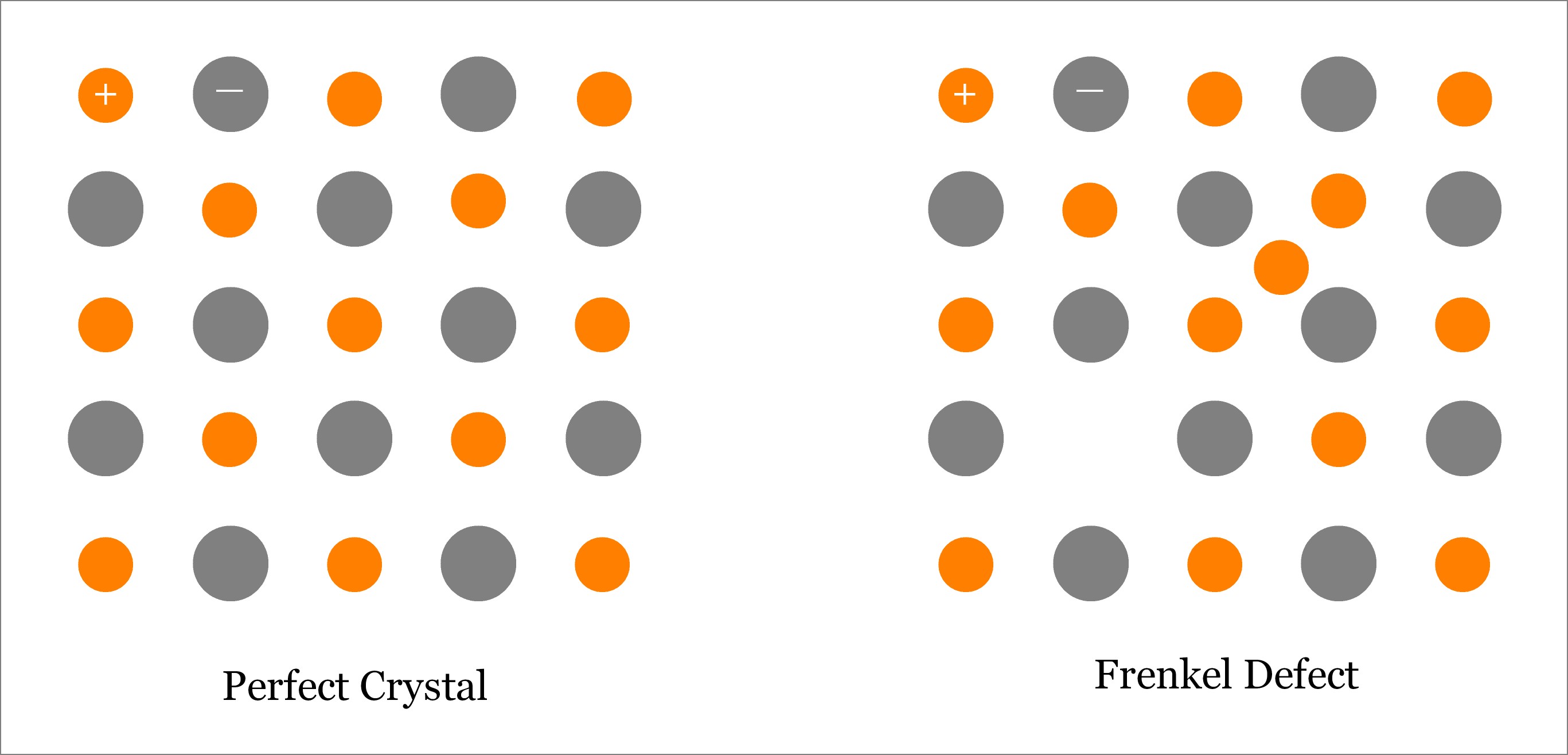
Frenkel Defect is one type of Point Defect; in fact, it is a combination of both Vacancy and Interstitial type of point defects. Usually, this type of defect is observed in ionic solids, where size of anion is substantially larger than the size of cation. Basically, a Frenkel Defect is one type of point defect where an atom (better to say ion, especially cation) leaves its original lattice site and occupies an interstitial position on the same crystal.
When an atom leaves the original lattice site then Vacancy Defect is created. Alternatively, when an atom (of same crystal or foreign crystal) occupies the interstitial site then Interstitial Defect is created. Since, in Frenkel Defect, one atom leaves the original lattice site and occupies an interstitial site in the same crystal, so Frenkel Defect is a pair of Vacancy & Self-interstitial Defects. The following image depicts a typical crystal with Frenkel Defect.
Example of Materials Where Frenkel Defect can be Found:
- Zinc Sulfide (ZnS)
- Silver Chloride (AgCl)
- Silver Bromide (AgBr)
Features of Frenkel Defect:
- In Frenkel Defect, neither any atom leaves the solid crystal nor do any foreign atoms occupy any interstitial position. So the overall density of the crystal solid remains unchanged.
- This type of defect is usually observed in ionic crystals, where size of anion is substantially larger than the size of cation. For example: zinc sulfide (ZnS), silver chloride (AgCl), etc.
- The smaller ions, usually cation, move from original lattice site to interstitial position; while, the larger ions (anion) remain in their corresponding lattice sites.
- It does not affect the chemical property of the solid.
- Electrical neutrality is also maintained in this defect.
Difference with Schottky Defect:
- Although both—Schottky and Frenkel defects occur in ionic materials, Frenkel defect occurs if size of anion is quite large as compared to that of the cation; whereas, Schottky defect occurs if difference in size between cation and anion is small.
- In Frenkel defect, only the smaller ion (cation) leaves its original lattice site; whereas, the anion remains in corresponding lattice sites. However, in Schottky defect, both cation and anion leave the solid crystal.
- Unlike Frenkel defect where one atom shifts from original lattice site to the interstitial position, in Schottky defect two atoms leave the solid crystal. So one vacancy and one self-interstitial occur in Frenkel defect; whereas, two vacancies occur in Schottky defect.
- The number of atoms present in the crystal before and after Frenkel defect remains same. However, one Schottky defect leads to the reduction of two atoms from the crystal.
- Density of the solid crystal before and after Frenkel defect remains same as no atom leaves the solid. However, Schottky defect reduces density of the solid.
- Read more: Difference Between Schottky Defect and Frenkel Defect.